PTT Permanganate Time Test
This method serves as a means of detecting the presence of impurities in alcohols or ketones that reduce potassium permanganate. Typically applied to Methanol, Ethanol, Propanol, Butanol, Acetone, Methyl Ethyl Ketone and Methyl Isobutyl Ketone.
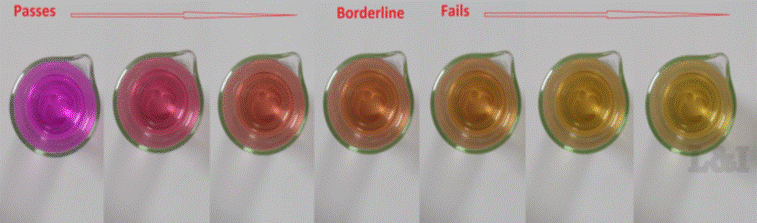
Substances reacting with potassium permanganate in neutral solutions reduce it to manganese dioxide, which colours the solutionfrom a pink colour to a yellow / brown colour.
In the permanganate test the time required for the colour of the test solution to change to that of a standard solution is measured.
The colour of the test solution changes from pink-orange to yellow-brown.
APPARATUS
- Cylinders, glass-stoppered, 50 ml tall form.
- Constant Temperature Bath, capable of maintaining a temperature of 15.0 ± 0.5 °C or 25.0 ± 0.5 °C
- Pipette, capable of delivering 2 ml of solution.
- Clock or stopwatch.
REAGENTS
- 0.02% solution of Potassium Permanganate., 0.100 g of KMnO4 per 0.5 liter water. This solution will oxidise in air and should be prepared freshly every 2 – 3 weeks. Storing in a fridge will help to prolong the life of the solution.
- Cobaltous Chloride – Platinum Cobalt standard Solution. (See ASTM D1363 for preparation details).This standard solution represents the colour of the end point to which the test sample solution fades. This solution is stable and should be kept in a 50 ml glass-stoppered cylinder, exactly the same as those in which the test is run.
PROCEDURE
- Fill a 50 ml glass-stoppered cylinder with the sample to be tested and place it in the constant-temperature-bath (15°C for Methanol or 25°C for Acetone), in order to allow the test sample to equilibrate at the appropriate temperature.
- When the sample has reached the bath-temperature (after about 5 minutes), add with a pipette 2 ml of the potassium permanganate solution.
- Stopper the tube, invert once to mix the contents, and return it to the bath.
- Determine the time from the addition of the potassium permanganate solution until the colour matches that of the standard. (see colour scale picture below)
- Protect the tube from light during this time. At the end of the test, clean the sample cylinders immediately with clean water otherwise they will quickly become stained with a brown colour. If this happens, fill the tubes with 10% hydrochloric or nitric acid and allow to stand until the tubes are visibly clean.
- Carefully remove the acid from the tubes and wash with copious amounts of tap water, followed by a final flush with DI water. NOTE: The presence of acid in the test cylinder during the permanganate test may cause the sample to fail prematurely.