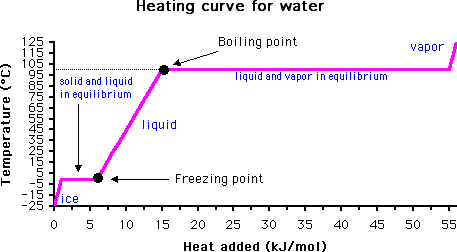
Boiling Point
Definition
Boiling point is the temperature at which a liquid changes to a gas (vapour) at normal atmospheric pressure.
A more specific definition of boiling point is the temperature at which the vapour pressure of a liquid is equal to the external pressure. The normal boiling point is the temperature at which the liquid boils when the external pressure is one atmosphere (760 torr = 760 mm Hg = 1 atm = 101.3 kPa = 14.7 psi).
More details
How can one determine the temperature at which water boils? After all, bubbles start to appear in water well below the known boiling point of 100 degrees C (212 F).
The answer lies in monitoring the temperature of the material with time. When the boiling point is reached, the temperature will not rise again until all of the liquid has evaporated. This is due to the high heat capacity of water (it takes much more energy to convert water from liquid to gas than it does to raise the temperature of liquid water).